Human extermination of species
Biodiversity is affected by many agents including overharvesting, introduction of exotic species, land use changes, nitrogen fertilization, and direct effects of increased atmospheric CO2 on plant ecophysiology. However, an overriding role of climate change is exposed by diverse effects of rapid warming on animals, plants, and insects in the past three decades.
A sudden widespread decline of frogs, with extinction of entire mountain-restricted species attributed to global warming, provided a dramatic awakening. There are multiple causes of the detailed processes involved in global amphibian declines and extinctions, but global warming is a key contributor and portends a planetary-scale mass extinction in the making unless action is taken to stabilize climate while also fighting biodiversity’s other threats.
Mountain-restricted and polar-restricted species are particularly vulnerable. As isotherms move up the mountainside and poleward, so does the climate zone in which a given species can survive. If global warming continues unabated, many of these species will be effectively pushed off the planet. There are already reductions in the population and health of Arctic species in the southern parts of the Arctic, Antarctic species in the northern parts of the Antarctic, and alpine species worldwide.
A critical factor for survival of some Arctic species is retention of all-year sea ice. Continued growth of fossil fuel emissions will cause loss of all Arctic summer sea ice within several decades.
IPCC reviewed studies relevant to estimating eventual extinctions. They estimate that if global warming exceeds 1.6℃ above preindustrial, 9–31 percent of species will be committed to extinction. With global warming of 2.9℃, an estimated 21–52 percent of species will be committed to extinction.
Hansen et al., 2013, Assessing ‘‘Dangerous Climate Change’’: Required Reduction of Carbon Emissions to Protect Young People, Future Generations and Nature.1
Seventeen years ago, in the mountains of Costa Rica, the Monteverde harlequin frog (Atelopus sp.) vanished along with the golden toad (Bufo periglenes). An estimated 67% of the 110 or so species of Atelopus, which are endemic to the American tropics, have met the same fate, and a pathogenic chytrid fungus (Batrachochytrium dendrobatidis) is implicated. Analysing the timing of losses in relation to changes in sea surface and air temperatures, we conclude with ‘very high confidence’ (> 99%, following the Intergovernmental Panel on Climate Change, IPCC) that large-scale warming is a key factor in the disappearances. We propose that temperatures at many highland localities are shifting towards the growth optimum of Batrachochytrium, thus encouraging outbreaks. With climate change promoting infectious disease and eroding biodiversity, the urgency of reducing greenhouse-gas concentrations is now undeniable.
Pounds et al., 2006, Widespread amphibian extinctions from epidemic disease driven by global warming.2
Despite increase of vegetation available for grazing, herd populations of caribou and wild reindeer across the Arctic tundra have declined by nearly 50% over the last two decades.
NOAA, 2018, Arctic Report Card.3
Among the most clear and profound influences of climate change on the world’s oceans are its impacts on habitat-forming species such as corals, sea grass, mangroves, salt marsh grasses, and oysters. Collectively, these organisms form the habitat for thousands of other species. Although some resident species may not have absolute requirements for these habitats, many do, and they disappear if the habitat is removed. For example, mass coral bleaching and mortality, the result of increasing temperatures, is already reducing the richness and density of coral reef fishes and other organisms.
Hoegh-Guldberg et al., 2010, The impact of climate change on the world’s marine ecosystems.4
It was not until the global bleaching event of 1982–1983 that widespread bleaching and mortality were recognised as a major phenomenon that could impact coral reef status and health at regional and global scales.
Oppen et al., 2018, Coral bleaching: patterns, processes, causes and consequences.5
Coral reefs provide some of the most biologically rich, productive and economically valuable ecosystems on Earth. Over 25 per cent of all marine species live in coral reefs, and yet they cover less than 0.1 per cent of the ocean, about half the area of France.
Globally, around 850 million people live within 100km of a coral reef and directly benefit from the economic, social and cultural services it provides. Reefs support many economically important fish species, providing food for hundreds of millions of people. They also protect the coast from storms and erosion, and generate jobs and income from fishing, tourism and recreation.
WWF, 2015, Living Blue Planet Report.6
The Great Barrier Reef – which, at 1,400 miles long, is the longest and largest coral reef in the world – was blanketed by dangerously hot water in the summer of 2016. This heat strangled and starved the corals, causing what has been called “an unprecedented bleaching event.”
The Atlantic, 2018, Since 2016, Half of All Coral in the Great Barrier Reef Has Died.7
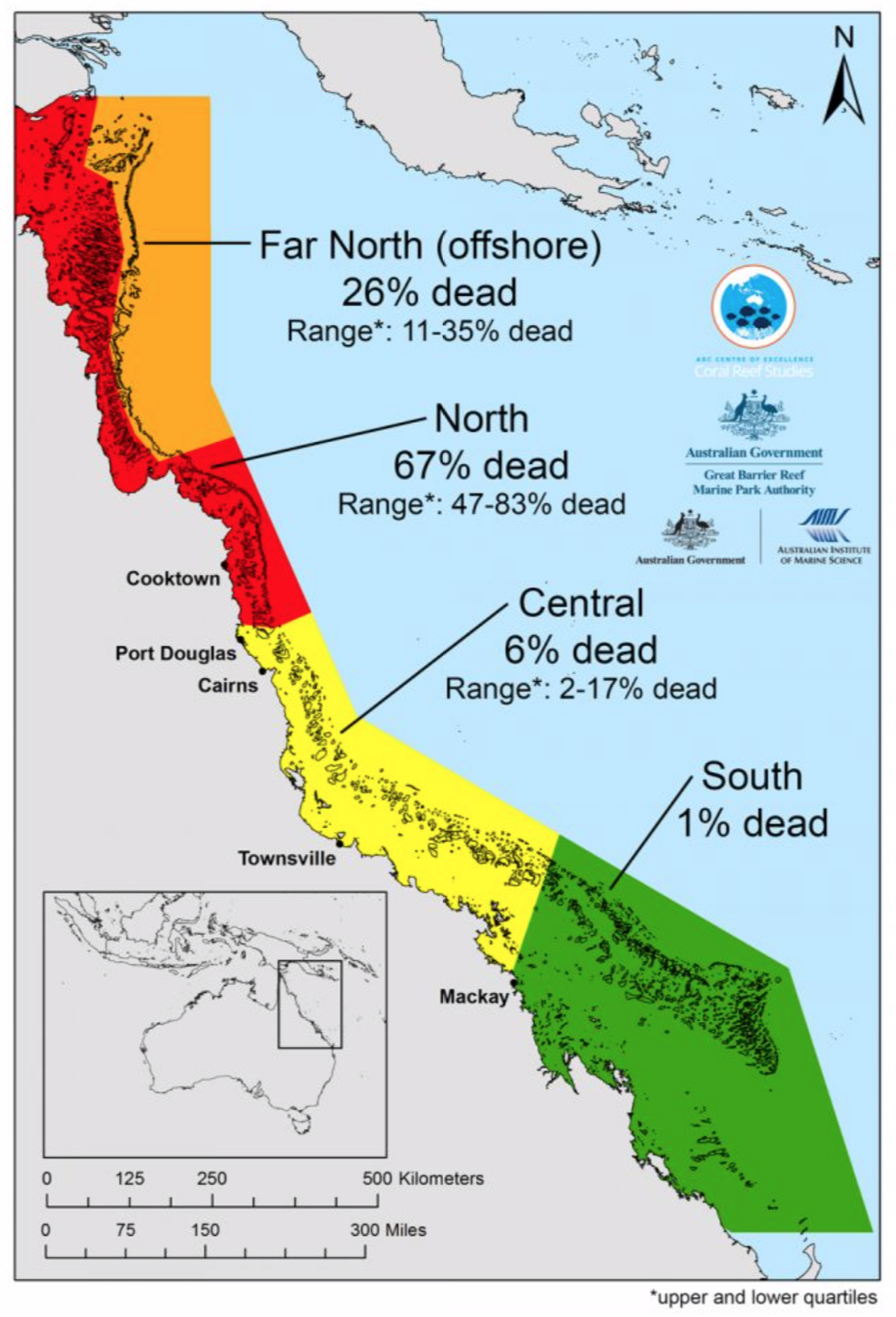
“On average, across the Great Barrier Reef, one in three corals died in nine months,” said Terry Hughes, an author of the paper and the director of the ARC Center of Excellence for Coral Reef Studies, the Australian government’s federal research program devoted to corals.
About 50 percent of all the coral that perished in the 2016 bleaching event died in the autumn and winter, long after temperatures had returned to normal. Those corals never regained their algae after evicting them, and they slowly starved to death.
“But about half of the corals that died did so in March, at the peak of summer temperatures,” Hughes told me. “We were surprised that about half of that mortality occurred very quickly.”
In other words, some corals did not even survive long enough to starve. “They died instantly, of heat stress,” Hughes said. “They cooked.”
Yet it was not the end of troubles for the Great Barrier Reef. In the summer months of 2017, warm waters again struck the reef and triggered another bleaching event. This time, the heat hit the reef’s middle third. Hughes and his team have not published a peer-reviewed paper on that event, but he shared early survey results with me.
Combined, he said, the back-to-back bleaching events killed one in every two corals in the Great Barrier Reef. It is a fact almost beyond comprehension: In the summer of 2015, more than 2 billion corals lived in the Great Barrier Reef. Half of them are now dead.
The study fits into a streak of dreary findings for coral reefs. If the world were to warm by an average of 2 degrees Celsius, then ocean temperatures would consistently exceed their 2016 levels, even during non-El Niño years, a recent study in Nature Climate Change found.9 In another study, released in January, scientists surveyed observations of 100 coral reefs around the world going back 35 years. They found that mass bleaching events now strike five times more often than they did in the early 1980s.10
The Atlantic, 2018, Since 2016, Half of All Coral in the Great Barrier Reef Has Died.7
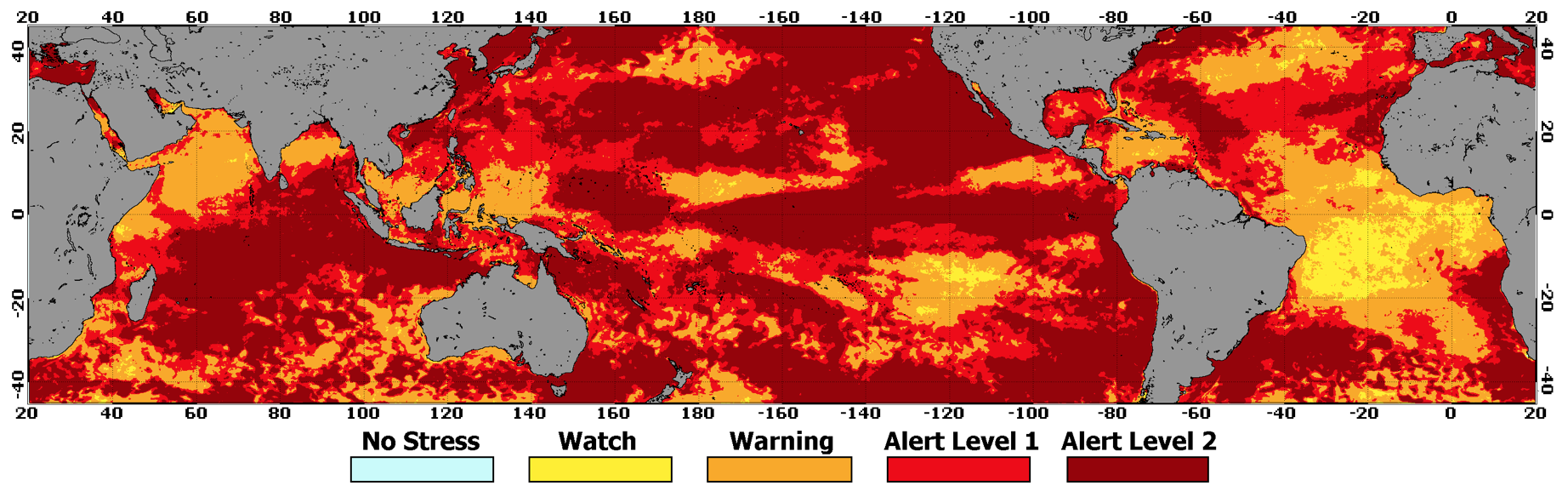
More than 70% of coral reefs around the world experienced the heat stress that can cause bleaching and/or mortality during the three-year long global event.
Multiple coral reef ecosystems around the world experienced severe bleaching in back–to–back years, including areas like Guam, where corals bleached every year from 2013 to 2017. As of the end of May 2017, the third global coral bleaching event most likely ended 12 but will remain the longest, most widespread, and possibly the most damaging coral bleaching event on record.13 It affected more reefs than any previous global bleaching event and was worse in some locales (e.g., Great Barrier Reef, Kiribati, Jarvis Island). Heat stress during this event also caused mass bleaching in several reefs that never bleached before (e.g., northernmost Great Barrier Reef).
Coral Reef Watch, NOAA, 2018.14
Recurrent regional-scale (>1000 km) bleaching and mortality of corals is a modern phenomenon caused by anthropogenic global warming. Bleaching before the 1980s was recorded only at a local scale of a few tens of kilometers because of small-scale stressors such as freshwater inundation, sedimentation, or unusually cold or hot weather.
Our findings reveal that coral reefs have entered the distinctive human-dominated era characterized as the Anthropocene, in which the frequency and intensity of bleaching events is rapidly approaching unsustainable levels.
At the spatial scale we examined, the number of years between recurrent severe bleaching events has diminished fivefold in the past four decades, from once every 25 to 30 years in the early 1980s to once every 5.9 years in 2016.
The time between recurrent events is increasingly too short to allow a full recovery of mature coral assemblages, which generally takes from 10 to 15 years for the fastest growing species and far longer for the full complement of life histories and morphologies of older assemblages.
Hughes et al., 2018, Spatial and temporal patterns of mass bleaching of corals in the Anthropocene.15
The summer of 2016 remains one of the most severe coral bleaching and die-off events ever observed14—a level of devastation that scientists didn’t expect to see until the 2050s. A new study argues that it will not remain a rare event for long. Even in simulations of the most hopeful global-warming scenarios, modern climate models suggest that ocean temperatures around the Great Barrier Reef will regularly surpass the devastating warmth of 2016.
In a 1.5-degree world, there’s still about a two-thirds chance that any summer would bring 2016-level heat to the Great Barrier Reef. And even in the coolest, kindest of the scenarios—the “gentlest” version of a 1.5-degree world—the odds of 2016-level heat are just over 50 percent. The reef would bleach every other year.
The Atlantic, 2017, The Great Barrier Reef Is Probably Doomed No Matter What.9
Marine heatwaves have also devastated kelp forests. “Kelp is a type of seaweed (or marine algae) that describes 27 genera worldwide. Some kelps form dense patches on rocky reefs resembling a forest of trees underwater and are referred to as kelp forests.”16

The first global study of kelp forest change over 50 years concluded that kelp had declined in 38% of the 1,138 sites studied globally.17
A hundred kilometres of kelp forests off the western coast of Australia were wiped out by a marine heatwave between 2010 and 2013, a new study has revealed.18
About 90% of the forests that make up the north-western tip of the Great Southern Reef disappeared over the period, replaced by seaweed turfs, corals, and coral fish usually found in tropical and subtropical waters.
The Great Southern Reef is a system of rocky reefs covered by kelp forests that runs along the south coast of Australia, extending past Sydney on the east coast, down to Tasmania and, previously, back up to Kalbarri on the west coast.
It supports most of the nation’s fisheries, including the lucrative rock lobster and abalone fisheries, and is worth about $10bn to the Australian economy. It is also a global biodiversity hotspot, with up to 30% of species endemic.
The Guardian, 2016, Australia’s vast kelp forests devastated by marine heatwave, study reveals.19
Today, more than 95 percent of eastern Tasmania’s kelp forests — luxuriant marine environments that provide food and shelter for species at all levels of the food web — are gone. With the water still warming rapidly and the long-spine urchin spreading southward in the favorable conditions, researchers see little hope of saving the vanishing ecosystem.
“Our giant kelp forests are now a tiny fraction of their former glory,” says Craig Johnson, a researcher at the University of Tasmania’s Institute for Marine and Antarctic Studies.20 “This ecosystem used to be a major iconic feature of eastern Tasmania, and it no longer is.”
The Tasmanian saga is just one of many examples of how climate change and other environmental shifts are driving worldwide losses of giant kelp, a brown algae whose strands can grow to 100 feet. In western Australia, increases in ocean temperatures, accentuated by an extreme spike in 2011, have killed vast beds of an important native kelp.
In southern Norway, ocean temperatures have exceeded the threshold for sugar kelp which has died en masse since the late 1990s and largely been replaced by thick mats of turf algae, which stifles kelp recovery.
In western Europe, the warming Atlantic Ocean poses a serious threat to coastal beds of kelp, and researchers21 have predicted “extirpation of the species as early as the first half of the 21st century” in parts of France, Denmark, and southern England.
YaleEnvironment360, 2017, As Oceans Warm, the World’s Kelp Forests Begin to Disappear.22
Ecology and the environment
The ecological impact of fossil fuel mining increases as the largest, easiest to access, resources are depleted. A constant fossil fuel production rate requires increasing energy input, but also use of more land, water, and diluents, with the production of more waste.
Coal mining has progressively changed from predominantly underground mining to surface mining, including mountaintop removal with valley fill, which is now widespread in the Appalachian ecoregion in the United States. Forest cover and topsoil are removed, explosives are used to break up rocks to access coal, and the excess rock is pushed into adjacent valleys, where it buries existing streams. Burial of headwater streams causes loss of ecosystems that are important for nutrient cycling and production of organic matter for downstream food webs. The surface alterations lead to greater storm runoff with likely impact on downstream flooding. Water emerging from valley fills contain toxic solutes that have been linked to declines in watershed biodiversity. Even with mine-site reclamation intended to restore pre-mined surface conditions, mine-derived chemical constituents are found in domestic well-water. Reclaimed areas have been found to produce little if any regrowth of woody vegetation even after 15 years, and, although this deficiency might be addressed via more effective reclamation methods, there remains a likely significant loss of carbon storage.
Oil mining has an increasing ecological footprint per unit delivered energy because of the decreasing size of new fields and their increased geographical dispersion; transit distances are greater and wells are deeper, thus requiring more energy input. Useful quantitative measures of the increasing ecological impacts are provided by the history of oil development in Alberta, Canada for production of both conventional oil and tar sands development. The area of land required per barrel of produced oil increased by a factor of 12 between 1955 and 2006 leading to ecosystem fragmentation by roads and pipelines needed to support the wells. Additional escalation of the mining impact occurs as conventional oil mining is supplanted by tar sands development, with mining and land disturbance from the latter producing land use-related greenhouse gas emissions as much as 23 times greater than conventional oil production per unit area, but with substantial variability and uncertainty. Much of the tar sands bitumen is extracted through surface mining that removes the ‘‘overburden’’ (i.e., boreal forest ecosystems) and tar sand from large areas to a depth up to 100 m, with ecological impacts downstream and in the mined area. Although mined areas are supposed to be reclaimed, as in the case of mountaintop removal, there is no expectation that the ecological value of reclaimed areas will be equivalent to predevelopment condition. Landscape changes due to tar sands mining and reclamation cause a large loss of peatland and stored carbon, while also significantly reducing carbon sequestration potential. Lake sediment cores document increased chemical pollution of ecosystems during the past several decades traceable to tar sands development and snow and water samples indicate that recent levels of numerous pollutants exceeded local and national criteria for protection of aquatic organisms.
Gas mining by unconventional means has rapidly expanded in recent years, without commensurate understanding of the ecological, environmental and human health consequences. The predominant approach is hydraulic fracturing (‘‘fracking’’) of deep shale formations via injection of millions of gallons of water, sand and toxic chemicals under pressure, thus liberating methane. A large fraction of the injected water returns to the surface as wastewater containing high concentrations of heavy metals, oils, greases and soluble organic compounds. Management of this wastewater is a major technical challenge, especially because the polluted waters can continue to backflow from the wells for many years. Numerous instances of groundwater and river contamination have been cited. High levels of methane leakage from fracking have been found, as well as nitrogen oxides and volatile organic compounds. Methane leaks increase the climate impact of shale gas, but whether the leaks are sufficient to significantly alter the climate forcing by total natural gas development is uncertain. Overall, environmental and ecologic threats posed by unconventional gas extraction are uncertain because of limited research, however evidence for groundwater pollution on both local and river basin scales is a major concern.
Hansen et al., 2013, Assessing ‘‘Dangerous Climate Change’’: Required Reduction of Carbon Emissions to Protect Young People, Future Generations and Nature.23
- p.7, Hansen J, Kharecha P, Sato M, Masson-Delmotte V, Ackerman F, et al. (2013) Assessing ‘‘Dangerous Climate Change’’: Required Reduction of Carbon Emissions to Protect Young People, Future Generations and Nature. PLoS ONE 8(12): e81648. doi:10.1371/journal.pone.0081648, https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0081648&type=printable[↩]
- Pounds, J.A., Bustamante, M.R., Coloma, L.A., Consuegra, J.A., Fogden, M.P., Foster, P.N., La Marca, E., Masters, K.L., Merino-Viteri, A., Puschendorf, R. and Ron, S.R., 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature, 439(7073), p.161, http://webpages.icav.up.pt/ptdc/bia-bec/099915/2008/9.Pounds%20et%20al%202006%20Nature.pdf[↩]
- https://www.arctic.noaa.gov/report-card[↩]
- Hoegh-Guldberg, O. and Bruno, J.F., 2010. The impact of climate change on the world’s marine ecosystems. Science, 328(5985), pp.1523-1528. https://www.researchgate.net/profile/Ove_Hoegh-Guldberg/publication/44683425_The_Impact_of_Climate_Change_on_the_World’s_Marine_Ecosystems/links/00b49516254ecbc78b000000.pdf[↩]
- Van Oppen, M.J. and Lough, J.M. eds., 2018. Coral bleaching: patterns, processes, causes and consequences (Vol. 233). Springer. https://books.google.com.au/books?hl=en&lr=&id=3DNjDwAAQBAJ&oi=fnd&pg=PR5&dq=Coral+Bleaching:+Patterns,+Processes,+Causes+and+Consequences&ots=thJLMEMXUa&sig=ppD7XBuAByjyKd1WaepvNqQxdbc#v=onepage&q=Coral%20Bleaching%3A%20Patterns%2C%20Processes%2C%20Causes%20and%20Consequences&f=false[↩]
- https://www.worldwildlife.org/publications/living-blue-planet-report-2015[↩]
- https://www.theatlantic.com/science/archive/2018/04/since-2016-half-the-coral-in-the-great-barrier-reef-has-perished/558302/[↩][↩]
- ARC Centre of Excellence for Coral Reef Studies, Media Release November 2016, https://www.coralcoe.org.au/media-releases/life-and-death-after-great-barrier-reef-bleaching[↩]
- https://www.theatlantic.com/science/archive/2017/05/the-great-barrier-reef-is-probably-doomed-no-matter-what/526641/[↩][↩]
- https://www.theatlantic.com/science/archive/2018/01/the-global-scourge-on-coral-reefs/549713/[↩]
- https://www.dropbox.com/sh/3vpwr5vi1ul6i9o/AADbaqwGq7Fg6TFqnzFnIGVEa?dl=0&lst=[↩][↩]
- http://www.noaa.gov/media-release/global-coral-bleaching-event-likely-ending[↩]
- http://www.noaa.gov/media-release/us-coral-reefs-facing-warming-waters-increased-bleaching[↩]
- https://coralreefwatch.noaa.gov/satellite/analyses_guidance/global_coral_bleaching_2014-17_status.php[↩][↩][↩]
- Hughes, T.P., Anderson, K.D., Connolly, S.R., Heron, S.F., Kerry, J.T., Lough, J.M., Baird, A.H., Baum, J.K., Berumen, M.L., Bridge, T.C. and Claar, D.C., 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science, 359(6371), pp.80-83, http://marinepalaeoecology.org/wp-content/uploads/2018/05/Hughes-et-al-Science-2018-with-cover.pdf[↩]
- https://www.nps.gov/chis/learn/nature/kelp-forests.htm[↩]
- http://www.imas.utas.edu.au/news/news-items/first-global-study-of-kelp-forest-change-gives-mixed-news-on-key-marine-ecosystem[↩][↩]
- http://science.sciencemag.org/cgi/doi/10.1126/science.aad8745[↩]
- https://www.theguardian.com/environment/2016/jul/07/australias-vast-kelp-forests-devastated-by-marine-heatwave-study-reveals[↩]
- http://www.imas.utas.edu.au/[↩]
- http://journals.plos.org/plosone/Read article?id=10.1371/journal.pone.0066044[↩]
- https://e360.yale.edu/features/as-oceans-warm-the-worlds-giant-kelp-forests-begin-to-disappear[↩]
- p.9, Hansen J, Kharecha P, Sato M, Masson-Delmotte V, Ackerman F, et al. (2013) Assessing ‘‘Dangerous Climate Change’’: Required Reduction of Carbon Emissions to Protect Young People, Future Generations and Nature. PLoS ONE 8(12): e81648. doi:10.1371/journal.pone.0081648, https://journals.plos.org/plosone/Read article/file?id=10.1371/journal.pone.0081648&type=printable[↩]